 Scans of the genome of patients with schizophrenia have revealed rare spontaneous copy number mutations that account for at least 10 percent of the non-familial cases of the disease. Researchers at Columbia University Medical Center describe specific genetic mutations present in individuals who have schizophrenia, but not present in their biological parents who do not have the disease. These individuals were eight times more likely to have these mutations than unaffected individuals. This new data, reported in the May 30 on-line issue of Nature Genetics, will help researchers account for the persistence of schizophrenia in the population despite low birth rates among people with the disease.
Scans of the genome of patients with schizophrenia have revealed rare spontaneous copy number mutations that account for at least 10 percent of the non-familial cases of the disease. Researchers at Columbia University Medical Center describe specific genetic mutations present in individuals who have schizophrenia, but not present in their biological parents who do not have the disease. These individuals were eight times more likely to have these mutations than unaffected individuals. This new data, reported in the May 30 on-line issue of Nature Genetics, will help researchers account for the persistence of schizophrenia in the population despite low birth rates among people with the disease.
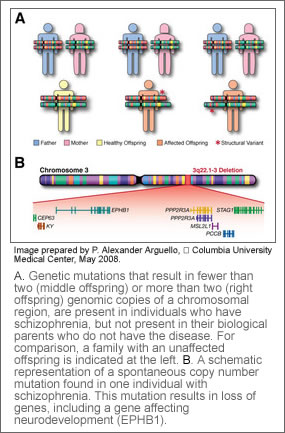
Researchers scanned the genome of 1,077 people which included 152 individuals with schizophrenia, 159 individuals without schizophrenia, and both of their biological parents for copy number mutations. They found mutations, either a gain or loss of genes, in 15 individuals diagnosed with schizophrenia that were not present in the chromosomes of either biological unaffected parent. Only two of such mutations were found in those without schizophrenia. Study subjects were from the European-origin Afrikaner population in South Africa, a genetically homogenous population that is ideal for genetic evaluation.
“We now know the cause of around 10 percent of the cases of sporadic schizophrenia,” said Maria Karayiorgou, M.D., professor of psychiatry, Columbia University Medical Center, the senior author on the study. “Schizophrenia is not as much of a ‘big black box’ as it used to be. The identification of these genes lets us know what brain development pathways are involved in disease onset, so that in the future we can look at better ways of treating this devastating disease.”
Schizophrenia affects approximately 1 percent of the population worldwide. About 40 percent of the disease is thought to be inherited, with the other 60 percent sporadically showing up in people whose family history does not include the disease.
One of the new or de novo mutations researchers found in more than one affected individual in this study was a deletion of a region of chromosome 22. Dr. Karayiorgou had previously provided evidence that loss of genes in this region, 22q11.2, was responsible for introducing “new” or sporadic cases of schizophrenia in the population. This confirms 22q11.2 as the only known recurrent such mutation linked to schizophrenia.
“We have already demonstrated 22q11.2 to be involved in sporadic schizophrenia and we have made considerable progress in understanding the underlying biological mechanisms,” said Dr. Gogos. “Now, we have a new set of mutations that we can investigate. The more information we have about the biological basis for this disease, the more information we can provide to those who suffer from it and their families.”
“Such abnormal deletions or duplications of genetic material are increasingly being implicated in schizophrenia and autism,” explains National Institute of Mental Health Director Thomas R. Insel, M.D. “Now we have a dramatic demonstration that genetic vulnerabilities for these illnesses may stem from both hereditary and non-hereditary processes. This line of research holds promise for improved treatments – and perhaps someday even prevention – of developmental brain disorders.”
Karayiorgou and co-senior author Joseph A. Gogos, M.D., Ph.D., associate professor of physiology and neuroscience at Columbia University Medical Center, agree that the goal is for psychiatrists to be able to inform patients that they have a mutation that is causing their disease and ultimately to be able to tailor treatments to individual patients based on their specific mutation. This tailored treatment is a ways off, according to Dr. Karayiorgou, but she says patients and their families are relieved to know that there is a biological cause of their illness.
The researchers plan to extend their screen for additional de novo mutations by using increased resolution scans to study additional families. They also plan to scrutinize further genes affected by the identified mutations through human genetics and animal model approaches.
The first author of this study, Bin Xu, is also from CUMC. Co-authors include J. Louw Roos from the Department of Psychiatry and Elizabeth J. van Rensburg from the Department of Genetics at the University of Pretoria in Pretoria in South Africa, and Shawn Levy from the Microarray Shared Resource at Vanderbilt University in Nashville, Tenn.
This study was supported by the National Institutes of Health’s (NIH) National Institute of Mental Health (NIMH) and the Lieber Center for Schizophrenia Research at Columbia University Medical Center.
Source: Columbia University, via Newswise
