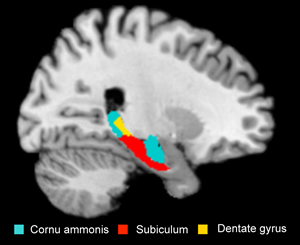
Depressive patients carrying the risk allele show volume reduction in certain regions of the hippocampus. Photo: MPI for Psychiatry
Max Planck scientists uncover surprising genetic links. Scientists from the Max Planck Institute for Psychiatry in Munich have compared the genomes of a total of 4,088 patients and 11,001 healthy control subjects from all over the world and identified a new risk gene variant for depression.
They were able to show for the first time that physiologically measurable changes can be observed in the brains of healthy carriers of this risk allele. Those changes affect a transporter protein involved in the production of an important neuronal transmitter. Given that traditional drugs interact with similar transporter molecules, the researchers are pinning great hopes on this factor as the target structure of future antidepressant medication. Scientists throughout the world have been trying to identify the genetic causes of depression for many years.
The fact that a search of this nature can involve exciting detective work is confirmed by Martin Kohli, Susanne Lucae, Bertram Müller-Myhsok and Elisabeth Binder in their current study. When comparing the genetic material of depressive patients with that of healthy control subjects, the researchers struck gold in a particular region on chromosome 12: they discovered individual base exchanges, so-called single-nucleotide polymorphisms (SNP), which clearly arose in the context of major depression. The researchers were very disappointed, however, when they had to establish that this section on the chromosome does not contain any genes. “Instead of getting our hands on a ‘depression gene’, we found ourselves back in a ‘genetic desert’ so to speak,” Florian Holsboer, Director of the Max Planck Institute for Psychiatry in Munich, explains.
Hence, the scientists wondered whether a gene located further away could possibly be influenced by the genetic variation and whether a susceptibility for depression could arise that way. The SLC6A15 gene was identified as a promising candidate for such a link. SLC6A15 contains the construction manual for a protein, which transports amino acids like proline and leucine to the contact sites of neurons in the brain, known as synapses, and may therefore be involved in the regulation of glutamate, an important neurotransmitter found in neurons. Leucine is a structural precursor of glutamate. “Because it is assumed that the communication between the neuron clusters is disturbed in depression, we considered whether the gene we had identified could possibly influence this process through glutamate,” explains Elisabeth Binder, research group leader at the MPI.
Indeed, the Max Planck scientists have succeeded in demonstrating that the changes in the DNA sequence located a total of 287,000 bases away from the gene influenced its activity: this varied in the brain samples examined, depending on which DNA components were exchanged. Therefore, cells of the risk genotype displayed a lower level of gene activity than cells carrying the protective genotype. Moreover, tests carried out by Philipp Sämann and his colleagues using the magnetic resonance imaging scanner confirmed that even healthy subjects who are carriers of the risk allele have smaller amounts of the brain metabolic substances N-acetylaspartate (NAA) and glutamate (Glx) compared to healthy subjects without genetic susceptibility.
Morphological changes also arise in the course of major depression: a shrinking of certain brain regions can eventually be observed in depressive patients. Therefore, in addition to genetic susceptibility, other factors must affect the organism to trigger the development of major depression. Research on the effects of stress as an important environmental factor in the development of depression has been carried out for many years at the Max Planck Institute for Psychiatry. It has been found, for example, that the likelihood of developing the disease increases by a factor of two to three if a person is exposed to chronic social stress.
In this context, an observation of Mathias Schmidt is of particular interest: he noted that the SLC6A15 activity in the brains of mice with an increased susceptibility to stress is considerably lower than in the brains of animals which are more resilient to stress. Irrespective of the associated cause and consequence, these data document the fact that there is a direct link between the examined gene and stress processing. “We would now like to find out how the gene converts stress into an activity-dependent cellular reaction and influences the disease process,” explains the young scientist and head of a Max Planck research group. The dependency of this gene activity on chronic stress provides impressive proof of the link between genetic and environmental influences at the molecular level in the disease process. Florian Holsboer firmly believes that “the detailed study of this stress-related gene regulation will give us a new understanding of the emergence of depression.”
Based on this discovery of the link between the transporter protein SCL6A15 and depression, the Munich scientists are cautiously optimistic that they are one step closer to the development of a new type of antidepressant. It has already been shown that tricyclic antidepressants can bind to a related transporter in bacteria and block its activity in this way. The extent to which the characteristics of the human SLC6A15 transporter can be influenced by small molecules and, based on this, an antidepressive effect can be generated is already being investigated by a new research project at the
Source: Max Plank Institute for Psychiatry
